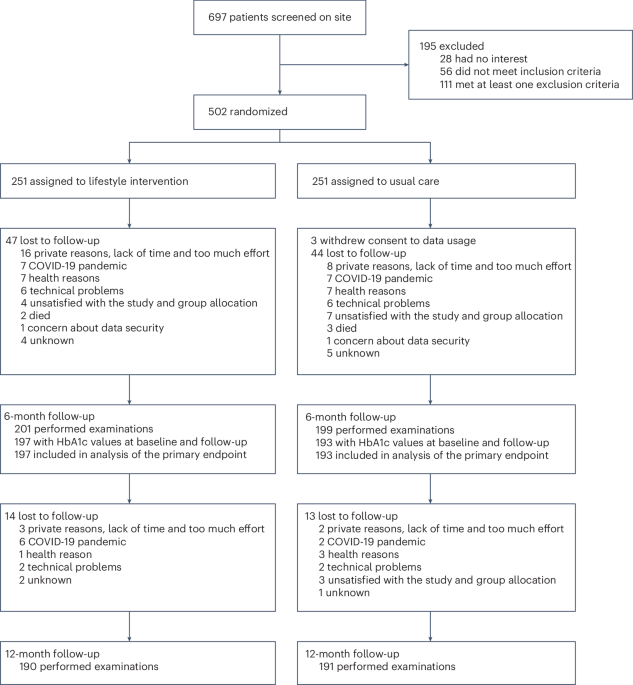
Study design and participants
LeIKD (ClinicalTrials.gov registration: NCT03835923) is a multicenter, randomized, clinical trial with two parallel groups conducted at 11 study sites in Germany (Munich, two sites in Berlin, Aachen, Magdeburg, Dresden, Leipzig, Kassel, Greifswald, Freiburg and Villingen-Schwenningen). All study participants were insured by one health insurance fund (Techniker Krankenkasse), which selected patients from their database according to the following criteria:
Diagnosis of CHD (International Classification of Diseases, Tenth Revision (ICD-10) code: I20–I25) and T2DM (ICD-10 code: E11; ICD-10 codes must have been reported at least two times to reduce the likelihood of false positive coding).
Enrollment in a disease management program for CHD and/or T2DM (including regular medical visits and offers for educational programs financed by the health insurance fund).
Living within 50 km of a participating study site.
Age ≥18 years.
Moreover, patients must have fulfilled none of the following criteria:
Mental and behavioral disorders (ICD-10 codes: F00, F01, F02, F11, F12, F13, F14, F15, F16, F18, F20, F21, F22, F23, F24, F25, F28, F29, F44, F72, F73, F17 and F84).
Heart failure New York Heart Association (NYHA) class IV (ICD-10 code: I50.14).
Malignant neoplasm (ICD-10 codes: C25, C34, C56, C72, C73, C78, C79 and C97).
Parkinson’s disease (ICD-10 code: G20).
Alzheimer’s disease (ICD-10 code: G30).
Infantile cerebral palsy (ICD-10 code: G80).
Chronic kidney disease (ICD-10 codes: N18.4 and N18.5).
Trisomy 21 (ICD-10 code: Q90).
Blindness/visual impairment (ICD-10 codes: H54.0, H54.2 and H54.3).
Hearing loss (ICD-10 codes: H90.0, H90.3, H90.5, H90.6 and H90.8).
Care level 1–5.
Insured abroad.
Patients who fulfilled the aforementioned criteria were contacted via telephone by an employee of the health insurance fund and informed about the possibility of getting screened for participation in the present trial. Contact details of interested patients were then forwarded to the study core lab in Munich and from there to the respective local study site to schedule the on-site screenings. During the on-site screening, patients were informed about all details of the study, provided their written informed consent and had to fulfill the following additional inclusion criteria:
Moreover, patients must have fulfilled none of the following criteria during on-site screening:
Inability to do physical exercises or conditions that may interfere with exercise intervention.
No optimal cardiac treatment within the last 4 weeks.
Not clinically stable within the last 4 weeks.
Participation in another clinical trial.
Randomization
Eligible patients were randomly assigned (1:1) to lifestyle intervention or usual care using a web-based system (secuTrial, interActive Systems GmbH) stratified by study site with block sizes of four. Patients and staff were not blinded to treatment group assignments.
Ethics approval
The original study protocol was approved by the ethics committee of the Technical University of Munich on 7 May 2018 (reference: 144/18 S) and the local ethics committees at the Universities of Berlin, Aachen, Magdeburg, Dresden, Leipzig, Greifswald and Freiburg, and the ethics committees of the Medical Associations of Hesse and Baden-Württemberg. A revised trial protocol (including a refinement of the inclusion criterion for the diagnosis of T2DM, that is, ‘HbA1c ≥ 6.5% or antidiabetic medication at the time of on-site screening’ in addition to ‘ICD-10: E11’) had also been approved by the ethics committees of the Technical University of Munich (reference: 144/18 S-AS) and the participating sites. All participants provided written informed consent. Detailed descriptions of the study design37 and baseline characteristics of the participants31 have been published previously and the study protocols, and statistical analysis plan can be found in the Supplementary lnformation.
Interventions
Each patient who was randomized to the lifestyle intervention group received an individualized exercise schedule including endurance (primarily walking and/or cycling, depending on patient preference) and strength training with the aim to progressively increase training duration to ≥150 min per week37. Endurance training intensities were based on ventilatory thresholds and peak oxygen consumption derived from cardiopulmonary exercise testing (CPET) and varied between low and moderate-to-vigorous intensities, including continuous and interval sessions (excluding high-intensity interval training). Strength training consisted of several exercises targeting different muscle groups, and each patient received a training booklet with figures, detailed instructions and multiple possible variations of each exercise, as well as access to a website with videos for each exercise. The individualized exercise training plan was provided via the LeIKD smartphone application (IDS Diagnostic Systems AG), which was also used to record training duration and intensity using a heart rate sensor (Polar H7, Polar Electro GmbH). These recordings provided the basis for regular individualized telephone feedback sessions. Patients were contacted in the first training week (third week after randomization), 2 weeks thereafter and then every 4 weeks to discuss their progress and potential adaptations to the exercise training plan. After 6 months, training intensities were adjusted based on the results of the repeated CPET, and patients received a final call to discuss their exercise training plan for the upcoming 6 months without further feedback.
The nutritional recommendations were based on 7-day nutrition diaries (including food and beverages), which patients had to complete in weeks 1, 5 and 14 after randomization, as well in week 1 after their 6-month follow-up. The diaries were evaluated based on the ‘energy density principle’, and each patient received individualized written feedback, including recommendations on how to improve eating patterns and the composition of their diet. Moreover, to increase health literacy and motivation, lifestyle intervention participants received e-mails twice weekly for 6 months with tips and tricks on how to increase daily physical activity and information on a well-balanced diet, including recipe recommendations.
The entire lifestyle intervention was centrally provided and communicated by the core study center in Munich. This included analysis of all CPET raw data, exercise recordings and nutrition diaries, sending e-mail newsletters and providing individualized telephone and written feedback on the exercise and diet intervention and adaptations.
In contrast, patients randomized to usual care did not receive active intervention but received one-time written standard recommendations on nutrition and physical activity according to current guidelines37. However, irrespective of group allocation, all patients received a pedometer (Beurer GmbH, AS80/AS87) and a blood glucose meter (Beurer GmbH, GL50evo) to monitor daily physical activity and blood glucose levels. These wearables were connected to a smartphone application (Beurer Health Manager; Beurer GmbH). If necessary, patients in either group received a smartphone for the duration of the study.
Conceptual framework of the intervention
The initial idea for the LeIKD trial stems from a healthcare program that is carried out in cooperation between the TUM University Hospital and the largest German health insurance fund, ‘Techniker Krankenkasse’ (Supplementary Table 1). Since 2011, more than 600 patients with CHD and/or T2DM have been included in this ongoing clinical program, which consists of a combination of supervised and home-based endurance and resistance training, nutritional counseling and coping and behavioral change strategies over 6 months. An analysis from a subsample of these patients has been published previously38.
The rationale of the LeIKD trial was to extend and transform this healthcare program to be broadly applicable. Given the funding by the Federal Joint Committee that decides which medical services are reimbursed by the statutory health insurance funds, the intervention was designed to be transferable into standard German healthcare. As on-site programs are neither sustainable nor cost-efficient in the long term, the program was designed as a home-based intervention with telemedical support (including individualized feedback from experts). The LeIKD smartphone application was adapted from an existing commercial application (mysportsapp; IDS Diagnostics Systems AG) that had been developed in cooperation with patients and experts at the TUM University Hospital and had already been applied in routine care for patients with cardiovascular diseases.
The intervention concept of the present study was further developed based on experiences from previously conducted large-scale exercise training trials in heart failure39,40,41, including one with telemedical support for the exercise training intervention40, as well as extensive institutional experience in prescribing exercise training and providing nutritional counseling for patients with metabolic and/or cardiovascular diseases in the routine care setting.
The exercise training program of the present trial started with a relatively high exercise training frequency (up to six times per week) and low session duration (as low as 10 min per session) to integrate regular exercise into everyday life. In adherence to the general exercise training principles of individualization and overload (including the secondary principles of variation and progression)42, the volume has then been gradually increased over time to reach at least 150 min per week. From the beginning, patients were regularly involved in the decision-making process on how the exercise plan could be adapted to better meet their individual needs. The high exercise frequency, the regular feedback, the shared decision-making and the additional health literacy training were also intended to improve self-efficacy, self-concordance, implementation intentions and volitional strategies to protect the behavior change against external and internal barriers, which are important elements in behavior change theories43.
The rationale of the nutritional counseling focusing on body weight loss in obese patients and overall dietary improvements was based on the energy density principle. This method has been shown to successfully reduce body weight with an individually tailored change of eating habits, has low barriers (as it does not prohibit any food) and is easily applicable and sustainable for patients with T2DM44. The energy density principle has also been frequently applied in the routine care of the coordinating study site and our aforementioned healthcare project in patients with CHD and/or T2DM38. Moreover, self-monitoring of dietary behavior and receiving regular feedback are considered behavior change techniques that are frequently applied in digital behavior change interventions45,46. Similar to the exercise intervention, the nutritional intervention has also been supported by additional health literacy information and recipe suggestions to promote a healthy and well-balanced diet.
Examinations and endpoints
All participants were assessed at baseline, 6 months after randomization and another 6 months after the first follow-up (12 months after baseline). Examinations included medical history, physical examination, anthropometry, electrocardiography, blood draw with local laboratory analysis, CPET and questionnaires to assess health literacy (European Health Literacy Questionnaire, HLS-EU-Q16), QoL (Short Form Health Survey, SF-36), daily physical activity (IPAQ) and eating behavior (Three-Factor Eating Questionnaire (TEFQ)).
Adverse events were documented by the local investigators and forwarded to the study core lab in Munich. Each adverse event form was reviewed by one physician blinded to treatment group assignment. If necessary, additional information (for example, hospital discharge letters) was requested from the local study sites. A serious adverse event was defined as any adverse event that resulted in death, was life-threatening, required hospitalization or prolongation of an existing hospitalization, resulted in persistent or significant disability or incapacity or required medical or surgical intervention to prevent a life-threatening condition, persistent or significant disability or incapacity. Major adverse cardiovascular events (4P-MACE) were defined as hospitalization due to angina/revascularization, nonfatal myocardial infarction, nonfatal stroke or cardiovascular death.
Primary endpoint was the change in HbA1c (%) after 6 months (analyzed in the local laboratories). Secondary endpoints included the following cardiovascular risk factors, QoL, health literacy, eating behavior, daily physical activity and safety parameters:
Change in HbA1c after 12 months (analyzed in the local laboratories).
Change in HDL cholesterol after 6 and 12 months (analyzed in the local laboratories).
Change in LDL cholesterol after 6 and 12 months (analyzed in the local laboratories).
Change in triglycerides after 6 and 12 months (analyzed in the local laboratories).
Change in body weight after 6 and 12 months (with validated weight standing scales).
Change in waist circumference after 6 and 12 months (with measuring tape).
Change in systolic blood pressure after 6 and 12 months (with validated blood pressure monitors, on the left upper arm after 5 min of rest).
Change in diastolic blood pressure after 6 and 12 months (with validated blood pressure monitors, on the left upper arm after 5 min of rest).
Change in health literacy after 6 and 12 months (HLS-EU-Q16 questionnaire; score range = 0–16 (higher scores reflect better health literacy)).
Change in QoL (physical component score) after 6 and 12 months (SF-36 questionnaire; score range = 0–100 (higher scores reflect better QoL)).
Change in QoL (mental component score) after 6 and 12 months (SF-36 questionnaire; score range = 0–100 (higher scores reflect better QoL)).
Change in eating behavior (cognitive restraint of eating) after 6 and 12 months (TFEQ; score range = 0–21 (higher scores reflect better cognitive restraint)).
Change in eating behavior (disinhibition) after 6 and 12 months (TEFQ; score range = 0–16 (higher scores reflect worse control)).
Change in eating behavior (hunger) after 6 and 12 months (TEFQ; score range = 0–14 (higher scores reflect higher susceptibility for internal and external hunger signs)).
Change in daily physical activity after 6 and 12 months (IPAQ).
Change in average steps per day over 7 days after 6 and 12 months (with pedometers).
Number of patients with 4P-MACE (including cardiovascular death, nonfatal stroke, nonfatal myocardial infarction and hospitalization due to angina or revascularization) after 6 and 12 months.
Adherence to the exercise intervention was measured using the exercise training recordings via the smartphone application and cross-validated by using the documentation of the regular feedback sessions. Adherence to the exercise intervention is reported as both median (IQR) minutes per week and the ratio between documented and prescribed exercise training time. Adherence to the nutritional intervention was measured by the ratio of completed to prescribed nutrition diaries. The additional secondary endpoints of changes in medical care expenses from baseline to 6 and 12 months are not reported in this manuscript and will be published separately. Likewise, the prespecified cost-effectiveness analysis will be published together with the medical care expenses.
Sample size calculation
For initial sample size calculation, a between-group difference in HbA1c of 0.4% with an s.d. of 1.8% (effect size d = 0.222) was assumed based on the results of the ‘Enhancing Adherence in Type 2 Diabetes (ENHANCE)’ trial47. With a power of 80%, a two-sided significance level of α = 0.05 and an estimated dropout rate of 15%, a total of 750 patients would have been required to detect a significant difference in the primary endpoint37. However, administrative delays, insufficient recruitment rates at several study sites and a fixed project duration required adjustments to the sample size calculation during the conduct of the study31. Based on a higher effect size of d = 0.305 with an estimated dropout rate of 30%, we aimed to include at least 486 patients31.
Treatment of missing data and loss to follow-up
To account for missing values of the primary endpoint, a multiple imputation approach has been applied. Imputation was performed using predictive mean matching (implemented in the R package ‘mice’)48 under consideration of the baseline parameters age, sex, BMI, HbA1c, insulin (yes/no), other antidiabetic medication (yes/no), CHD classification (≤1 vessel disease versus ≥2 vessel disease), education level (low/medium versus high), usage of mobile applications (daily versus less than daily) and randomization group. Ten datasets with imputed values were generated and pooled to test the null hypothesis of equal change in HbA1c for both groups.
Statistical analysis
All endpoints except for the number of patients with 4P-MACE were evaluated with t tests for independent samples, whereas the number of patients with 4P-MACE was evaluated with the χ2 test. All patients were analyzed in the group they were randomized to. For the main analyses, only patients with complete paired baseline and follow-up measurements were included. However, the analysis of the primary endpoint (change in HbA1c at 6 months) was repeated after multiple imputations of missing values as specified in the statistical analysis plan and described above. For the prespecified per-protocol analysis, patients of the lifestyle intervention group were excluded from the analysis when they had completed <66.7% of the planned overall exercise duration or achieved the planned weekly exercise duration in <50% of weeks or completed <2/3 of the nutrition diaries until the 6-month follow-up. To evaluate the robustness of the results from the per-protocol analysis, two additional post hoc analyses were performed. First, the criterion for performed/prescribed exercise training time was increased to ≥100%. Second, the criteria for performed/prescribed exercise training time and filled nutrition diaries were increased to ≥100% and three of three nutrition diaries. To confirm the robustness of the findings from the per-protocol analysis, the point estimates for the primary and secondary endpoints of these post hoc analyses should be comparable to or more favorable than in the original per-protocol analysis. Moreover, the analysis of the primary endpoint was repeated within prespecified subgroups by fitting linear regression models with tests for interaction between these variables and study groups to the data. All statistical tests were performed using R Statistical Software (v.4.0.2, R Foundation for Statistical Computing) with two-sided significance levels of α = 0.05. As CIs have not been adjusted for multiple testing, analyses of secondary endpoints should be interpreted as exploratory.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.