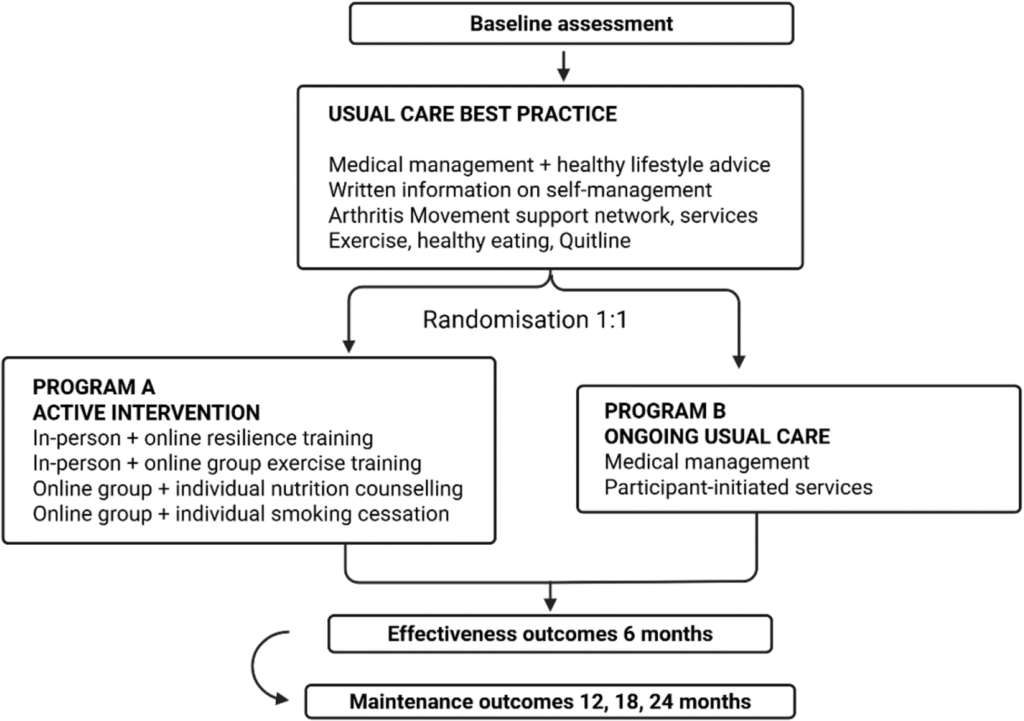
Explanation for the choice of comparators {6b}
Both the European League of Rheumatology EULAR and the American College of Rheumatology (ACR) recommend that patients with rheumatic diseases are educated about the benefits of managing stress and lifestyle improvements including a healthy diet, regular exercise and encouragement to quit smoking (21, 22). The comparator group will receive usual best practice care, which incorporates the provision of advice on recommended lifestyle modifications.
Intervention description {11a}
Study arms: all participants
At the baseline assessment, all participants will receive written lifestyle advice information sheets from Arthritis Australia (Arthritis and Emotional Wellbeing; Exercise and RA; Healthy Eating and Arthritis; Physical Activity Arthritis Information Sheet; 10 Steps For Living Well With Arthritis) (23), as well as from the Australian Rheumatology Association (ARA) (Things You Can Do to Manage Your Rheumatoid Arthritis (24)); The Quitline for smoking cessation, and The Arthritis Movement contact details for support and exercise programmes (20), with questions answered verbally by the PI. All participants will receive a diary in which to record adverse events, such as injuries.
Study arms: programme A—RA-heal intervention
Programme A participants will take part in the RA-HEAL intervention. It will be delivered over 5 months, comprising five partially overlapping multidisciplinary person-centred interventions designed to recognise individual preferences, values, and concerns; with shared decision making, empowerment, and physical and emotional support, with some basic resources provided.
Programme A will be delivered as a combination of one-on-one or group in-person and videoconference sessions (Fig. 2). In-person sessions will be delivered at four sites (Princess Alexandra Hospital, Ipswich Hospital, the University of Queensland St Lucia campus, and Griffith University Nathan campus), with oversight from A/Prof Nicola Burton (CHP), Dr Hannah Mayr (DN), Prof Jeff Coombes (EP, and Prof Coral Gartner (tobacco treatment specialist). Participants will be able to select their preferred in-person group location and time from the multiple time options offered and will be encouraged to attend the sessions at the same site throughout the intervention phase. Online and in-person groups will be capped at 10 people.
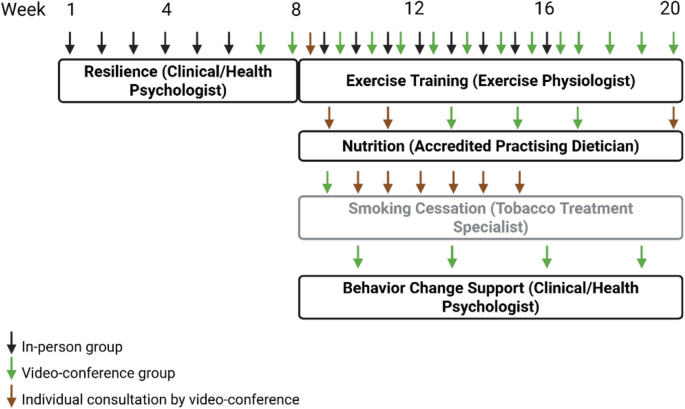
Participants will complete the full intervention schedule of resilience, exercise training, nutrition, smoking cessation and behaviour change support over a 20-week period.
Multidisciplinary Intervention 1: Resilience
This group component is scheduled at the beginning to introduce key wellbeing concepts over the first eight weeks of the intervention. Each week participants will attend both an onsite group session and an online group session. It aims to provide support on adjustment to RA and to provide a foundation for coping strategies relevant across the other components. Drawing from Acceptance and Commitment Therapy, Cognitive Behavior Therapy and Behavior Activation principles, sessions will focus on the role of mental health in RA self-management, mindfulness, acceptance, cognitive flexibility, coping strategies, life values and meaningful actions, social support and sleep. Sessions will include information giving, personal reflection, experiential activities, skill rehearsal, feedback and problem-solving and applied practice “homework”. A written handbook on session content and activities will be provided.
Multidisciplinary Intervention 2: Exercise Training
The twelve-week exercise training component will commence in week 9. Initially, a 1-h individual session will determine participants’ exercise capacity, muscular fitness, exercise goals, and preferences. Their individualised exercise prescription will be based on medical history, interests/preferences, physical capacity and suitability for self-direction. Participants will then attend group exercise training sessions, within a mixture of weekly onsite and online group sessions. Each session will target recommendations of ≥ 150 min/week of moderate to vigorous aerobic and muscle strengthening exercise. Aerobic exercise will focus on walking unless significant lower limb musculoskeletal impairment prevents walking or stationary cycling, in which case suitable alternatives will be identified based on participant capabilities and access to resources. Strength exercises will involve upper and lower limbs using body weight and provided Therabands and small weights for use on site and at home. A Fitbit will also be provided to participants to facilitate self-monitoring.
Multidisciplinary Intervention 3: Nutrition
The nutrition intervention will also commence in week 9 and will run over twelve weeks. Initially, a 1-h individual participant consultation will assess nutrition and set personalised goals. This will be followed up with two further 30-min individual consultations and also three 1-h group sessions that provide nutrition education and practical strategies related to swapping or replacing foods, food preparation and recipes, label reading, shopping and eating out. Counselling will recommend alignment with principles of a Mediterranean-style diet (25, 26), including promoting higher intake of foods with anti-inflammatory properties (vegetables, fruits, nuts and seeds, legumes, fish and seafood, whole grain breads and cereals, herbs/spices, extra virgin olive oil), moderate intake of unflavoured dairy foods, poultry and eggs, and lower intake of pro-inflammatory or ultra-processed foods including processed meats, red meat, sugary drinks, and other packaged or commercial foods and drinks high in saturated fat, sugar and/or sodium. Recommendations will be foods-focused to promote overall diet quality without a specific target macronutrient profile. The recommendations will be individualised and tailored; evidence-based resources and recipes which align with the Mediterranean-style diet principles (e.g. from Queensland Health Nutrition Education Materials Online and National Heart Foundation) will be provided where appropriate.
Multidisciplinary Intervention 2: Smoking Cessation
Participants who smoke will also commence a seven-week smoking cessation programme, in week 9. Initially, a group education session will be offered. This will be followed by weekly individual consultations. Sessions will include nicotine dependence assessment, quitting self-efficacy, lung age calculator, individual Quit Plan, education, support, and practical coping strategies. Over-the-counter stop smoking Nicotine Replacement Therapy options will be recommended and demonstrated. Dosing is based on World Health Organization “first line therapy” and Royal Australasian College of General Practitioners guidelines, including combination patches plus oral nicotine replacement, which is one of the most successful approaches to quit smoking (27). Prescription Varenicline and Bupropion options will be supported in line with participant preferences, in consultation with the participant’s usual GP.
Multidisciplinary Intervention 4: Behavior Change Support
Behavior change support sessions will be conducted during the final month of the intervention, starting in week 17. These sessions will be delivered by the same clinical/health psychologist who provided resilience training in week 1 of the intervention. They will focus on integrating wellbeing and healthy lifestyle activities into everyday life, and responding to related challenges. Content is based on the 5 A Counselling Framework, which is an evidence-based approach appropriate for chronic conditions and endorsed by the RACGP and US Task Force on Preventive Health Care. The key components of the 5 A framework include:
1.
Assessment: change experiences/barriers/enablers
2.
Advice: clear recommendations for change
3.
Agree: Collaborative agenda setting
4.
Assistance: Cognitive and behavioural change strategies as indicated, e.g. motivational interviewing, goal setting, action planning, positive expectations, problem-solving, self-monitoring, social support, self-talk
5.
Arrange: Additional assistance/resources as relevant.
At the completion of the intervention period, participants will be invited to attend an online focus group to discuss intervention experiences and associated barriers and enablers to engagement in the interventions.
Study arms: programme B—ongoing best practice usual care
Participants in Programme B will receive brief monthly check-ins by email to inform of appointment times for follow-up and to monitor AEs. Participants will be referred to their general practitioner or rheumatologist should they need further management.
Criteria for discontinuing or modifying allocated interventions {11b}
N/a.
Explanation
This study does not trial any medicinal products and as detailed in the above intervention description, all components of the multidisciplinary intervention will be personalised to accommodate individual participants’ needs.
Strategies to improve adherence to interventions {11c}
One of the barriers to programme adherence is the significant time commitment required. Details have been provided at informed consent and will be discussed verbally during screening. A support person (e.g. family member or friend) is encouraged to be at the consent meeting to assist with information retention. Letters to the participant’s treating rheumatologist and general practitioner will encourage support from other members of the health care team. The person-centred approach used in Programme A (e.g. individualised exercise training and nutrition) is intended to take into account individual concerns and preferences and empower participant participation. Offering options for different times and locations for in-person interventions, including weekends, as well as some sessions via videoconference to reduce travel burden, is intended to facilitate adherence. Commencing with resilience is intended to provide intrapersonal strategies to assist with engagement to the lifestyle component.
Relevant concomitant care permitted or prohibited during the trial {11d}
Participation in RA-HEAL is considered an adjunct to a participant’s continuous medical care from their usual rheumatologist (once stable on conventional or biological DMARDs, appointments are typically every 3–6 months), general health care with their usual general practitioner and any additional support services and activities (including related to healthy eating, exercise, smoking cessation, wellbeing) initiated by them before or during the intervention period.
Provisions for post-trial care {30}
After the trial, all participants will continue to receive RA treatment with their usual rheumatologist, general health care with their usual general practitioner, and any additional support services and activities initiated by them.
Outcomes {12}
The primary outcome is physical health-related QoL at 6 months, as determined by the SF-36 v2 physical component score.
Secondary outcomes include:
Physical health-related QoL: as well as the primary outcome of testing at 6 months, SF-36 v2 physical component score at 12, 18 and 24 months will also be measured
Confidence for self-management of RA: the Perceived Competence Scales will assess confidence in taking an active role in managing wellbeing, exercise, healthy eating, medications and (if relevant) smoking cessation in the context of having RA at 6, 12, 18 and 24 months.
Physical function and anthropometry: exercise capacity (6-Minute Walk Test (6MWT)), neuromuscular strength (grip strength with a dynamometer), power (Timed-Up & Go (TUG) test) and strength endurance (5 times sit to stand test and 30 s arm curl test), gait speed (4 m walk test) and balance (single leg balance test); height, weight and waist circumference at 6, 12, 18 and 24 months.
Mental health: SF36v2 mental health component score, the Warwick-Edinburgh Mental Wellbeing Scale and the Perceived Stress Scale at 6, 12, 18 and 24 months.
Physical activity: physical activity and sedentary behaviour will be assessed; (i) objectively via Actigraph accelerometry over one week at baseline and at 6 months, and (ii) by self-report using items from the National Health Survey at 6, 12, 18 and 24 months (28).
Diet quality: the 50-item Mediterranean Diet and Culinary Index (MediCul) at 6, 12, 18 and 24 months, to provide a score between 0 and 100 (29). The tool also allows for calculation of a commonly reported validated 14-item Mediterranean diet adherence score (30).
Smoking cessation: self-report at 6, 12, 18 and 24 months, (7-day and continuous abstinence), including number of cigarettes smoked, nicotine dependence, quitting self-efficacy, i.e. self-confidence about making another quit attempt (if quit attempt was unsuccessful); biochemically verified exhaled carbon monoxide.
RA disease activity score (DAS28CRP): a composite measure of patient global score, tender and swollen joint counts and C-reactive protein, at 6, 12, 18 and 24 months. DAS-remission will be defined as a DAS < 2.4. Remission duration will be defined as the length of time over which a DAS < 2.4 is measured.
Cardiovascular disease risk: will be measured using the Australian CVD Risk Calculator (31), which calculates CVD risk percentiles based on input variables.
Overall health-related quality of life: Assessment of Quality-of-Life score 8 Dimension (AQoL-8D) score
We will evaluate the 5 components of Reach, Effectiveness, Adoption, Implementation, Maintenance from the (RE-AIM) framework in the RA-HEAL study.
Reach refers to eligible people willing to engage with programmes. It will be measured firstly as the number of people who consent to the trial as a function of the number of queries from eligible potential participants. Participant representativeness will compare sociodemographic, lifestyle and clinical profile of the trial participants with the A3BC database of RA participants with RA onset in the last 12 months. Reach in each arm will be assessed as the proportion of participants recruited to the study who (i) in programme A engage with each component (resilience, exercise training, nutrition, smoking cessation, and behaviour change) and (ii) in programme B seek engagement with professional services related to mental health, exercise, nutrition or smoking cessation support for RA, including through The Arthritis Movement programmes or private service providers.
Effectiveness refers to short-term impact. It will be measured as the change from baseline at 6, 12, 18 and 24 months using the measures specified in the primary and secondary outcome measures.
Adoption refers to an agency’s use of an intervention. It will be measured as:
Number and characteristics of staff implementing the programme.
Implementation refers to the delivery of the intervention. It will be measured as:
1.
Fidelity: An online checklist from multidisciplinary interventionists will be used to assess the consistency of Programme A delivery (e.g. number of sessions provided, content covered) against written protocols. Provision of written information resources to participants will be recorded.
2.
Feasibility:
a.
The proportion of participants completing each component of and the entire programme A relative to the number allocated to and commencing programme A, and
b.
The proportion of participants engaging with Programme A sessions as a function of the total number of sessions for each of the Programme A components.
3.
Acceptability:
a.
Participant feedback on satisfaction with and perceived helpfulness of their programme and resources for RA self-management.
b.
Programme A intervention experiences, and associated barriers and enablers to engagement across the various component sessions.
Cost effectiveness: Modified cost diaries capturing health resources accessed, supplemented by Medicare Benefit Schedule (MBS) and Pharmaceutical Benefit Scheme (PBS) data. Health resources will be valued using market prices and industrial wages. Utility scores to allow quality-adjusted life years (QALYs) gained will be obtained using SF-6Dv2, a subset of SF36v2 using Australian weights. An exploratory analysis will compare the performance of SF-6Dv2 and AQoL-8D.
Maintenance refers to sustained change and will compare the primary and secondary effectiveness measures at 6, 12, 18 and 24 months.